Academy
24.02.2026
South Korea shows interest in programs supporting young scientists
Read more CloseThe SAMS General Secretariat met with a South Korean delegation visiting Bern to attend the Korea-Switzerland Joint Committee on Science and Technology meeting. The discussion focused on the Academy’s funding programs for young medical researchers.
Myriam Tapernoux, Head of the Funding division at the SAMS, presented the Swiss National MD-PhD Fellowships Program and the Young Talents in Clinical Research (YTCR) funding program, which encourages young physicians to take their first steps in clinical research, to a delegation from the Ministry of Health and Welfare and the Korea Health Industry Development Institute. The Koreans showed great interest and invited the SAMS to present its activities at a conference. All information about our funding activities is available on our website.
Visit the website
Funding
19.02.2026
Call for proposals 2026: National MD-PhD program
Read more CloseThis year, the SAMS is once again awarding competitive MD-PhD fellowships to talented young physicians to complete a doctoral research training in natural sciences, clinical research, public health sciences or biomedical ethics. Thanks to the partnerships with foundations and associated faculties, the national MD-PhD program can be continued. The submission deadline for applications is 15 May 2026.
The National MD-PhD Program fellowships are intended for candidates admitted as doctoral students in the local MD-PhD program at one of the partner universities. The evaluation takes place in two stages: The National MD-PhD Committee evaluates the applications and selects the candidates to be interviewed. The Committee then submits a funding recommendation to the SAMS Senate and the supporting foundations. The list of partner faculties and foundations, the program FAQ, and all details on submission can be found on our website.
Visit the website
Academy
17.02.2026
Save the date: Symposium on choices for our health system
Read more CloseThe SAMS has long been committed to the sustainable development of the Swiss health system. Within this context, the Academy is organizing a symposium on 24 September 2026 at the Eventforum Bern, focusing on the choices our society will have to make in the near future regarding our health system in order to address the growing human, financial, and environmental constraints.
During the symposium on 24 September 2026, the following questions, among others, will be discussed: Which decisions lie ahead? How can outcomes be measured meaningfully? How can high-value care be promoted? How can the risk of rationing be avoided? What role do patients and citizens play in the decision-making processes? Be sure to mark 24 September 2026 in your calendar. Information on registration and the program will be announced in our newsletter and on our website where you can already find background information on the topic.
Further information
SPHN
16.02.2026
Viktor Award 2025: Vote now for SPHN
Read more CloseThe Swiss Personalized Health Network (SPHN) is one of five organizations nominated for the Viktor Award 2025 in the category «Pioneering Achievement in the Swiss Healthcare System». By voting for SPHN, you are recognizing the commitment of a large community of university and cantonal hospitals, universities, and research institutions throughout Switzerland.
Launched by the Swiss Confederation in 2017, SPHN is under the responsibility of the SAMS in collaboration with the SIB Swiss Institute of Bioinformatics. SPHN's mission is to provide a data infrastructure for research with health data. There are at least three good reasons why SPHN deserves the Viktor Award: SPHN laid the foundations for data-driven medicine in Switzerland, connected a fragmented ecosystem and delivers lasting impact for patients and the healthcare system. Public voting is open until 2 March 2026.
Visit the website
Projects
12.02.2026
CPCR Service Finder for clinical research: a new tool to access services across organizations
Read more CloseThe CPCR Service Finder, a new collaborative, user-friendly tool, has been developed by the national Coordination Platform Clinical Research (CPCR) with the technical expertise of Swiss Biobanking. It allows to browse among services from the main national research infrastructures, and to identify whom to approach for support, in one click.
As host of the CPCR, the SAMS is pleased to announce the launch of the CPCR Service Finder. It is a first tangible outcome of the CPCR – whose mission is to foster synergies among organizations conducting and supporting clinical research in Switzerland – the CPCR Service Finder helps academic researchers, study coordinators, data managers, and biobank professionals identify which national infrastructures can provide relevant expertise, data, or resources at different stages of their projects.
Visit the website
Funding
06.02.2026
Call for applications: For Women in Science Program
Read more Close«For Woman in Science» is the result of the cooperation between UNESCO and L'Oréal. Since 1998, these two partners have been promoting women in science, fostering gender equality and highlighting the visibility of women in research as role models. The Swiss Program was launched in 2025 in partnership with the Swiss Academy of Engineering Sciences (SATW). Following last year's success, the partners continue the program with the 2026 edition.
For this second edition, the program will award a total of CHF 100’000 distributed in 4 endowments of CHF 25’000 each to postdoctoral female researchers conducting exceptional work in science, technology, engineering, and mathematics (STEM) in Switzerland. The call for applications is also open to medical professionals. Registration is possible until 15 March 2026. Further information can be found on the SATW website.
Visit the website
Projects
04.02.2026
smarter medicine: Top 5 list on headaches published
Read more CloseThe association «smarter medicine – Choosing Wisely Switzerland», of which the SAMS is a member, is committed to providing optimal healthcare in Switzerland. It encourages professional societies to draw up Top 5 lists defining interventions that should be avoided altogether or only used under specific conditions. In February 2026, the Swiss Headache Society (SHS) published such a list.
The SHS makes the following five recommendations: Generally not advisable are repeated MRI scans for headaches which do not evolve, CT scans for non-acute symptoms, tooth extractions for persistent facial pain, the removal of amalgam fillings for pain treatment, and migraine surgery. The aim is to avoid unnecessary examinations and risky therapies and to improve treatment based on evidence. You can download the SHS Top 5 list here (in French); all other lists are available on the smarter medicine website (in French or German).
Top 5 list, French version (PDF)
SPHN
03.02.2026
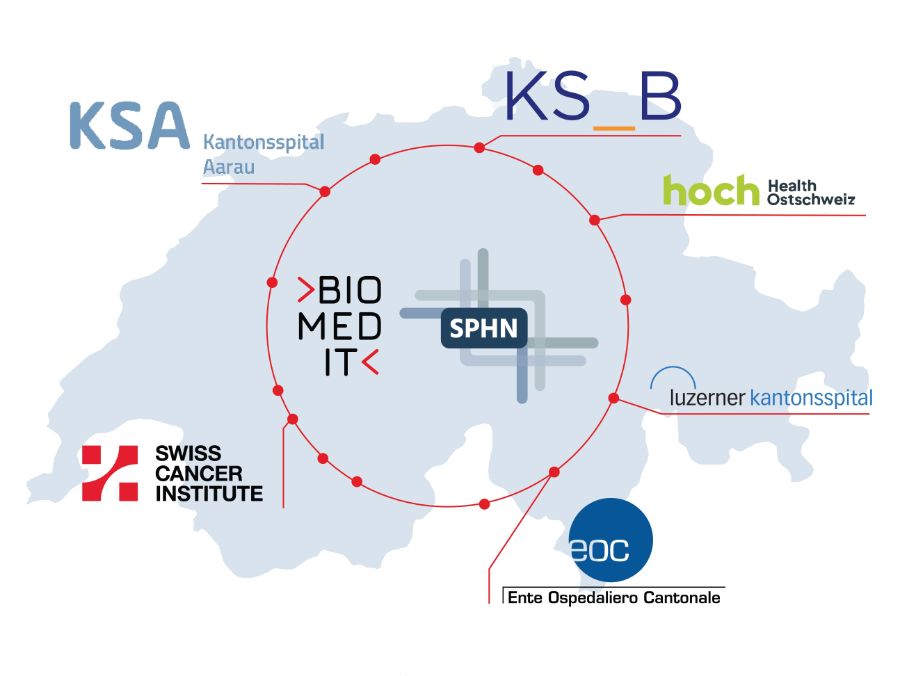
New healthcare institutions onboarded to SPHN
Read more CloseSix new healthcare institutions have been onboarded to the Swiss Personalized Health Network (SPHN). These include five cantonal hospitals and the Swiss Cancer Institute, which are now ready to contribute clinical data to multi-site research projects in personalized medicine. This is a key achievement in the ongoing effort to extend and standardize health data exchange across Swiss healthcare institutions.
The newly onboarded institutions currently provide – to various degrees – a standardized minimal dataset covering demographics, diagnoses, procedures, medications, laboratory tests, etc. The connection to the Swiss Cancer Institute further strengthens oncology research. Now, standardized datasets from Swiss cancer patients can be shared in the SPHN format. These developments are paving the way for collaborative, data-driven research and ultimately, for more personalized and effective patient care. More Information can be found on the SPHN website.
Visit the website